Page 563 from
Start-Up Activity
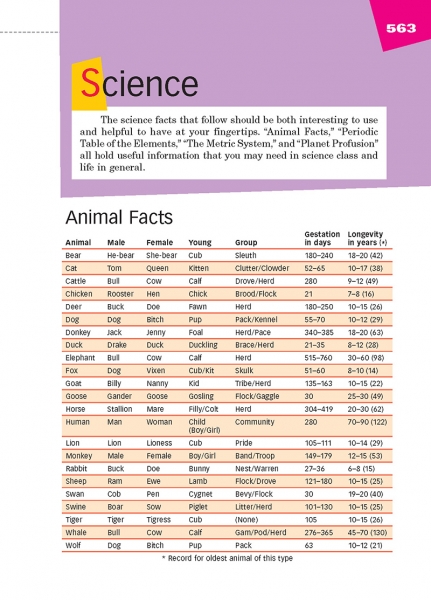
Have students review the animal facts on this page. Direct their attention especially to the fifth column, which names groups of animals. Point out some of the more interesting groups:
-
A clutter of cats
-
A skulk of foxes
-
A bevy of swans
Then acquaint students with a few other interesting group names:
-
A shrewdness of apes
-
A cauldron of bats
-
A murder of crows
-
A business of ferrets
-
A flamboyance of flamingos
-
A conspiracy of lemurs
-
A scurry of squirrels
Now challenge students to come up with their own names for groups of people: kindergartners, teachers, politicians, doctors, Goths, jocks, and so on. For example, they might suggest
- An argument of lawyers
- A suit of lawyers
- A pinstripe of lawyers
Think About It
“What do you call a smiling, courteous person at a convention of lawyers? The caterer.”
—Anonymous

Start-Up Activity
Have students review the animal facts on this page. Direct their attention especially to the fifth column, which names groups of animals. Point out some of the more interesting groups:
-
A clutter of cats
-
A skulk of foxes
-
A bevy of swans
Then acquaint students with a few other interesting group names:
-
A shrewdness of apes
-
A cauldron of bats
-
A murder of crows
-
A business of ferrets
-
A flamboyance of flamingos
-
A conspiracy of lemurs
-
A scurry of squirrels
Now challenge students to come up with their own names for groups of people: kindergartners, teachers, politicians, doctors, Goths, jocks, and so on. For example, they might suggest
- An argument of lawyers
- A suit of lawyers
- A pinstripe of lawyers
Think About It
“What do you call a smiling, courteous person at a convention of lawyers? The caterer.”
—Anonymous